Five-letter words and legal language

As the climate talks in Paris came to a conclusion this past December, some contention arose around the use of a five-letter word: “shall.” Whether rich countries “shall” or “should” contribute money for poor countries’ transitions may seem like a trivial difference, but it was certainly not “just semantics” to U.S. negotiators. In international agreements, “shall” implies a binding legal requirement, and Secretary of State John Kerry made it clear that unless “shall” became the much squishier “should,” the world’s largest economy would have no part in the most serious climate change resolution to date.
{mosads}As important as “shall” may be in international diplomacy, its significance in domestic matters should not be underestimated. While “shall” is most often used as a synonym for “will” in common conversation, in legal writing, the word is closer in meaning to “must.” In fact, Congress has even passed laws that explicitly acknowledges the strength of these restricting terms, as well as others, in various contexts. For instance, in Title 21 of the Code of Federal Regulations (the title in which the Food and Drug Administration [FDA] publishes its regulations), clear guidelines are enumerated to keep these potent words out of intentionally weak “guidance documents” for regulations:
Guidance documents must not include mandatory language such as “shall,” “must,” “required,” or “requirement,” unless FDA is using these words to describe a statutory or regulatory requirement.
While the general public might not care so much about whether regulators use “shall” or “must” in legal language, the specific intent with which they use these restrictions offers a useful proxy to measure how many regulatory requirements exist overall. This was the approach that was used in the creation of RegData, a free database that quantifies federal regulation by industry, by agency and over time.
One statistic that RegData produces is a measure of how restrictive specific sections of regulatory text are, and to do this, RegData counts up occurrences of restrictions like “shall” and “must.” We took a look at the 2014 Code of Federal Regulations to see just how common these words actually are in regulations. As it turns out, both the words “shall” and “must” are extremely common in federal regulations — to the point that they are the first and third most common words in the entire 2014 Code of Federal Regulations (excluding articles like “the” and “a” and section headers, like “Sec.”). The table below lists the top 10 most common words and the number of times we found each of them.
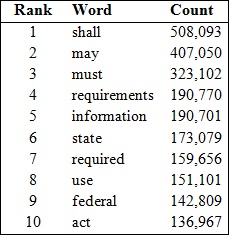
The thousands of new instances of “shall,” “must” and other binding restrictions that enter federal regulatory code each year accumulate over time. And this accumulation of regulatory restrictions can have negative consequences: At some point, so many cumulated restrictions can impede judicious risk management, stifle innovation and ultimately stunt economic growth.
Thus, it makes sense that regulators and businesses often care a great deal about legal word choice; clarity is key when the economic consequences — either positive or negative — are this high. Yet despite its frequent usage in regulations, “shall” is actually not the best word for regulators to use when making an obligation or prohibition. While the FDA uses the term to indicate regulatory requirements, the Federal Aviation Administration has sided against the word, telling its own lawyers, “If you mean mandatory, write ‘must.'” Detracting further from the word’s consistency, in Gutierrez de Martinez v. Lamagno, the U.S. Supreme Court ruled that “shall” can actually mean “may” in certain contexts.
Legal writing expert Bryan Garner advocated in 2012 for “shall” to be deleted entirely from legal prose:
In most legal instruments, shall violates the presumption of consistency: Words are presumed to have a consistent meaning in clause after clause, page after page. Which is why shall is among the most heavily litigated words in the English language (with hopelessly inconsistent court holdings).
Instead, the word “must” is preferred, because it is not ambiguous. And some agencies have issued formal internal guidance to their own lawyers, instructing them that they must not use “shall” when creating legally binding obligations or prohibitions.
When billions of dollars of regulatory costs are on the line, it’s important to pay attention to these semantic arguments over five-letter words. However, if proposals such as Garner’s can circumvent these arguments altogether, they might make the labyrinthine regulations and laws more transparent and accessible, allowing the public to focus on the substance of the rules instead.
McLaughlin is a senior research fellow with the Mercatus Center at George Mason University. Jares is a student at the University of Rochester, where he is studying economics and mathematics.
Copyright 2023 Nexstar Media Inc. All rights reserved. This material may not be published, broadcast, rewritten, or redistributed. Regular the hill posts








